BACKGROUND: Human herpesvirus-8-negative/idiopathic multicentric Castleman disease (iMCD) is a heterogenous group of diseases characterized by proinflammatory hypercytokinemic state with a wide range of systemic manifestations ranging from generalized lymphadenopathy to death in severe cases. Limited data has shown an increased prevalence of organ dysfunction and cancers in iMCD patients either as a result of underlying disease pathophysiology or treatment received. The objective of this study was to assess the healthcare resource utilization, patterns of iMCD-related comorbidities, and survival of iMCD patients in a real-world setting.
METHODS: We performed a retrospective analysis of administrative claims data for >30 million United States patients continuously enrolled over a three-year study period from January 1, 2017 and December 31, 2019. Patients were identified as iMCD if they had an ICD-10 diagnosis code for Castleman disease (D47.Z2) and ≥2 diagnostic codes corresponding to the minor criteria from the international evidence-based consensus diagnostic criteria for iMCD. Exclusion criteria included history of HIV, HHV-8, lymphoma, myeloma, lupus, or rheumatoid arthritis within one year of diagnosis of Castleman disease. Index diagnosis date (IDD) was defined as the first time a patient received a diagnosis for Castleman disease using the new ICD-10 code (D47.Z2) or the general code for lymphadenopathy (785.6) in ICD-9 that included Castleman disease, whichever was diagnosed first between 2006 and 2019. Included patients were followed for up to 5 years from IDD and evaluated for post-diagnosis hospitalizations, emergency room visits, hematologic malignancies, non-hematologic malignancies, thromboses, and organ dysfunction. Estimated average survival was calculated based on estimated incidence and prevalence rates for the iMCD patient population, assuming a stable incidence.
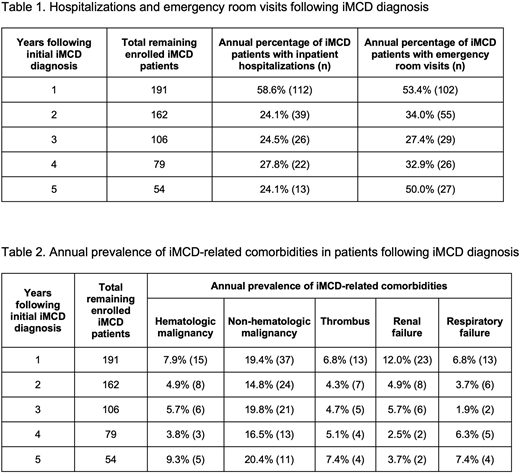
RESULTS: We identified 191 iMCD patients including 109 women (57%) and 82 men (43%) with a mean age of 51 years (range: 6 - 90). The average post-diagnosis follow up was 3.2 years after IDD (range: 0.3 - 14.1). Within the first year of iMCD diagnosis, 58.9% of patients required inpatient hospitalization and 53.4% had at least one emergency room visit. Among the patients who remained enrolled after the first year, an average of 25.1% were hospitalized and 36.1% visited the emergency room during each subsequent year (Table 1). The annual rate of hospitalizations and emergency room visits for the entire database of >30 million patients was 9.0% and 20.6%, respectively. As shown in Table 2, the annual prevalence of iMCD-related comorbidities in this cohort was 18.2% for non-hematologic malignancies, 6.3% for hematologic malignancies (including lymphomas and myelomas from second year follow up onwards), 5.8% for thromboses, 5.7% for renal failure, and 5.2% for respiratory failure. Based on an average annual incidence of 2.45 (95% CI, 0.85 - 8.60) per million and average prevalence of 6.31 (95% CI, 3.25 - 13.05) per million, we estimated an average survival of 2.57 (95% CI, 1.59 - 4.25) years after diagnosis in this study time period.
DISCUSSION: Using a large nationally representative health claims database, we identified a cohort of iMCD patients and found a high rate of hospitalizations and emergency room visits in the first five years following diagnosis. The annual prevalence of iMCD-related organ failure was approximately 5-6% primarily involving renal and respiratory systems. This study provides further evidence to support the previously reported increase in frequency of subsequent hematologic and non-hematologic malignancies in iMCD patients. In the five-year follow up period, the average estimated survival of iMCD patients was 2.57 (95% CI, 1.59 - 4.25) years after diagnosis, which is shorter than previously published average survival durations based on academic medical centers. The worse survival in this cohort may represent iMCD survival outside of academic medical centers or may reflect inaccuracies in estimated incidence and prevalence which were used to estimate average survival. Further studies are needed to compare outcomes to age-matched controls and to determine whether these adverse outcomes are broadly seen across iMCD patients or instead attributable to a smaller subset of more severe cases.
Mukherjee:Bristol Myers Squib: Honoraria; Partnership for Health Analytic Research, LLC (PHAR, LLC): Honoraria; Celgene: Consultancy, Honoraria, Research Funding; Aplastic Anemia and MDS International Foundation: Honoraria; Novartis: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; EUSA Pharma: Consultancy; Celgene/Acceleron: Membership on an entity's Board of Directors or advisory committees. Martin:EUSA Pharma: Current Employment. Sande:EUSA Pharma: Current Employment. Glen:HVH Precision Analytics: Current Employment. Paige:HVH Precision Analytics: Consultancy. Yarlagadda:HVH Precision Analytics: Current Employment. Fajgenbaum:EUSA Pharma: Research Funding; Janssen Pharmaceuticals: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal